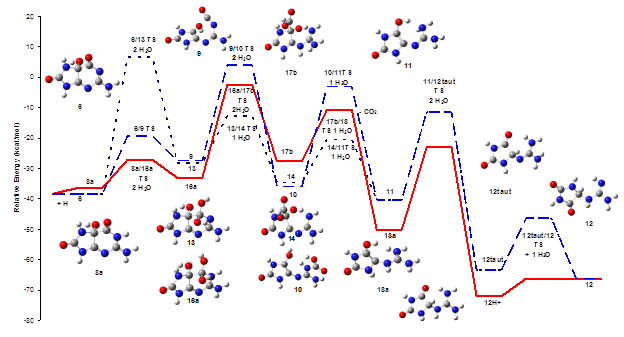
Conversion of 5-hydroxy-8oxoguanine to
guanidinohydantoin
Oxidative damage of DNA is closely related to
mutagenesis, cancer, toxicity and aging. Because of the numerous interconnected
pathways and the fleeting nature of the intermediates, oxidative damage to DNA
is difficult to study experimentally. We have used computational chemistry
explore the reaction mechanisms and elucidate the properties of the short-lived
intermediates in guanine oxidation in collaboration with Prof. Cynthia Burrows
at U. of Utah. Large changes in solvation energy on removal of a proton or an
electron make it challenging to calculate pKa’s and redox potentials for
biochemically relevant oxidation processes.
We have
developed a protocol using continuum solvation models with cavity scaling to
calculate reliable pKa’s and redox
potentials for nucleobases and applied it to study the pKa’s
and redox potentials of nucleobases and intermediates in the oxidative degradation
of guanine.
a.
Munk, B. H.; Burrows, C. J.; Schlegel, H.
B.; An Exploration of Mechanisms for the Transformation of 8-Hydroxy Guanine
Radical to FAPyG by Density Functional Theory. Chem. Res. Toxicol.
2007, 20, 432-444 (10.1021/tx060187t).
b.
Munk, B. H.; Burrows, C. J.; Schlegel, H.
B.; Exploration of Mechanisms for the Transformation of 8-Oxoguanine to Guanidinohydantoin and Spirodihydantoin
by Density Functional Theory. J. Am.
Chem. Soc. 2008, 130, 5245-5256
(10.1021/ja7104448).
c.
Psciuk, B. T.; Lord, R. L.; Munk, B. H.; Schlegel, H. B.; Theoretical Determination of
One-Electron Oxidation Potentials for Nucleic Acid Bases. J. Chem. Theory Comput. 2012, 8, 5107−5123 (10.1021/ct300550x).
d.
Psciuk, B. T.; Schlegel, H. B.;
Computational Prediction of One-Electron Reduction Potentials and Acid
Dissociation Constants for Guanine Oxidation Intermediates and Products. J. Phys. Chem. B 2013, 117, 9518–31 (10.1021/jp4062412).